Kinetics
Chemical Kinetics is the study of chemical reactions with respect to the rate of reaction, and other factors that affects this speed.
This is especially useful for determining how a reaction occurs.
Activation Energy
Consider the reaction:
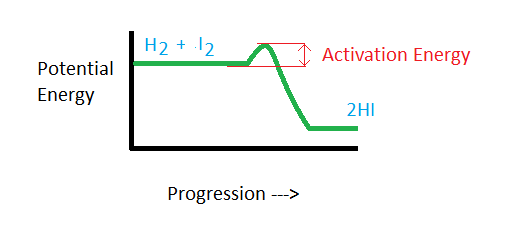
H2 + I2 → 2HI
The point where the H-H, and I-I bonds break and reform is known as the high energy state, or the activation state. After the initial breaking and reforming, the H-I molecules form, which is in a lower energy state.
A positive catalyst increases the rate of reaction by lowering the activation energy level.
Increasing the temperature will increase the rate of reaction as the molecules reach the activation state much quicker.
Increasing the Surface Area also increases the rate of reaction as more atoms are available to react.
High activation energy means the reaction rate will be slower as more energy is required to cause the reaction to occur.
The activation energy of a chemical reaction is closely related to its rate. The higher the activation energy, the slower the reaction will occur.
This is because the molecules can only complete the reaction once they have reached the top of the activation energy barrier.
Factors That Affect Kinetics (Reaction Rates)
1. Concentration
- There has to be sufficient of each reactants
2. Temperature
- Higher temperature = faster rate of reaction
- Lower temperature = less energy = slower rate of reaction
3. Phase/State, and Surface Area
- If both are in the same phase (for example, both are liquids) /state, it usually helps the speed of the reaction along
4. Solvent
- For example, if the solvent can hydrogen-bond with one of the products, this will reduce the reactivity of the bonded molecule.
- Viscosity also plays a part. An increase in the viscosity of the solvent will reduce the reaction rate.
5. Catalyst
- Increase the chemical reaction rate, without undergoing net chemical change itself
- Higher concentration means there are more molecules/particles present to react with. Just like surface area
- However, if one of the reactants is concentrated beyond the point of excess, the rate will not increase further (not get any faster)
There also exists catalysts that slow reactions down.
Rate Equation
Rate = Change in Concentration / Time
The result is always positive, and in mol/seconds.
A + B → Products
| [A] | Initial Rate | [B] | Initial Rate |
|---|---|---|---|
| 1M | 0.01 m/s | 1M | 0.01 m/s |
| 2M | 0.02 m/s | 2M | 0.04 m/s |
| 3M | 0.03 m/s | 3M | 0.09 m/s |
| ↑ [A] × 2x = ↑ Rate × 2 | ↑ [B] × 22 = ↑ Rate × 4 |
| ↑ [A] × 3x = ↑ Rate × 3 | ↑ [B] × 32 = ↑ Rate × 9 |
| ∴ R ∝ [A]1 | ∴ R ∝ [B]2 |
R = K [A]1 [B]2
- R = Rate of reaction
- K = Rate constant
- [A]1 = **First** order in A (the 1)
- [B]2 = **Second** order in A (the 2)
Overall order = 3
So, to find K:
m/s = K (m)1 (m)2
m/s = K (m)1 (m)2
∴ K = 1 / (m2 × s)
K changes depending on overall order
Rate Law/Equation Example
2NO (g) + 2H (g) → N2 (g) + 2H20 (g) *(@ 1280°C)
| Experiment | [NO] (M) | [H2] (M) | Initial Rate (M/s) |
|---|---|---|---|
| 1 | 0.0050 | 0.0020 | 1.25×10-5 |
| 2 | 0.0100 | 0.0020 | 5.00×10-5 |
| 3 | 0.0050 | 0.0040 | 1.00×10-4 |
First, determine the rate law:
R = K [NO]? [H2]?
In experiments #1 and #2, the concentration of [NO] increased × 2, and the Rate increased 4 fold (400%, or × 4).
∴ 2x = 4
∴ x = 2
R = K [NO]2 [H2]? …this 2 shows second order
In experiments #2 and #3, the [H2] goes up × 2, and the Rate increases × 2 also.
∴ 2y = 2
∴ y = 1
R = K [NO]2 [H2]1
Question: What is the overall order of the reaction?
Answer: 3 (2 order + 1 order)
Question: Find the rate constant K?
Answer: You can just plug in the values to one of the experiments above:
1.25×10-5 M/s = K [0.005 M]2 [0.002 M]1
1.25×10-5 M/s= K (5×10-8 M2 × M)
K = 250 1/M2×s @ 1280°C
Question: What is the rate of reaction when the concentration of NO ([NO]) is 0.012M and the [H2] is 0.0060M
Answer: R = K [NO]2 [H2]1
R = 250 1/M2×s [0.012 M]2 [0.0060 M]1
R = 250 1/M2×s [0.000144 M2 × 0.0060 M]
R = 250 1/M2×s [8.64×10-7 M2 × M]
R = 0.000216
R = 2.16×10-4 M/s





You must be logged in to post a comment.