Isomers (Part 2 of 2)
You can find the first part of this series here: Isomers (Part 1 of 2).
Chirality
- A molecule is chiral if it is not superimposable on it’s mirror image
- Most chiral molecules can be identified by their lack of a plane, or centre of symmetry
- Your hand for example, is a chiral object
- The most common cause of chirality in a molecule is an atom that is bonded to four different groups
- The atom that has 4 different groups is called the stereocenter
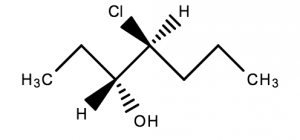
This molecule has 2 chiral centres. Two of the atoms have 4 different groups bonded to them
A chiral molecule that has stereocentres can have multiple three dimensional representations. In general, a chiral molecule with stereocenters has 2n different forms; where n = number of stereocenters in the molecule.
Diastereomers
Diastereomers are stereoisomers that are not mirror images of one another and are non-superimposable on one another.
- Not mirror images of one another ✓
- Non-superimposable ✓
∴ They are diastereomers
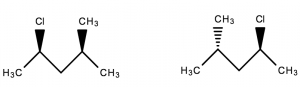





You must be logged in to post a comment.