Acids and Bases (1 of 2)
Part 2 of Acids and Bases is located here.
Definition of pH
Typically the hydrogen ion concentration of a solution is expressed in terms of pH.
pH is calculated as the negative log of a solutions hydrogen ion concentration
pH = -log10[H+]
If you plug the hydrogen ion concentration of water into this equation ([H+] of water = 1×10-7 M) you get 7. This is known as neutral pH.
- High concentration of [H+] ions means a low pH (acidic)
- Low concentration of [H+] ions means a high pH (basic)
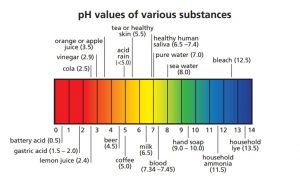
The pH Scale
Bronsted-Lowry
- Acid: Proton (H+) donor
- Base Proton (H+) acceptor
HCl + H2O → Cl- + H3O+
- In this reaction an extra electron from the H-Cl bond moves to the Cl, making it a Cl–, and leaving the hydrogen as a H+
- The H2O then acts as a Bronsted-Lowry base by accepting a proton (the H+)
- So in terms of Bronsted-Lowry, the acid is HCL, and the base is the Water
- This means the conjugate base is the Cl– and the conjugate acid is H3O+
pKa and pKb Relationship (Weak Acids and Bases)
First, note that square brackets indicate the concentration of. For example: [H+] denotes the concentration of the hydrogen ion.
Now, in relation to weak acids and bases:
Ka = [products] / [reactants]
and:
Kb = [products] / [reactants]
Note also, this is only true for reactions in equilibrium. This means it can only be done for weak acids, or bases.
For example:
HA (aq) ↔ H+ (aq) + A– (aq)
∴ Ka = [H+] [A–] / [HA]
And:
Kb = [HA] [OH–] / [A–]
-log Ka + -log Kb = 14
- -log Ka = pKa
- -log Kb = pKb
(aq) meaning it is an aqueous solution, which means dissociating in water
Weak Acid Equilibrium
A = Acid, for demonstration purposes
HA + H2O ↔ H3O+ + A–
Ka = [H3O+] [A–] / [HA] [H2O]
Example; with weak acids:
Note again that to calculate the pKa = -log Ka , and the lower the pKa value, the stronger the acid:
| Ka | pKa | ||
| HF | Hydrofluoric Acid | 3.5 x10-4 | 3.46 |
| CH3COOH | Acetic Acid | 1.8 x10-5 | 4.74 |
| CH3OH | Methanol | 2.9 x10-16 | 15.54 |
Weak Base Equilibrium
B = Base for demonstration purposes
B + H2O ↔ BH+ + OH–
Kb = [BH+] [OH–] / [B]
pKb = -log Kb
| Kb | pKb | ||
| NH3 | Ammonia | 1.8 x10-5 | 4.74 |
| C6H5NH2 | Aniline | 4.3 x10-10 | 9.37 |
The lower the pKb value, the stronger the base.





You must be logged in to post a comment.