Isomers (Part 1 of 2)
You can find the second part of this series here: Isomers (Part 2 of 2).
Isomers may also be called Structural Isomers, or Constitutional Isomers.
Isomers are molecules that have the same molecular formula, but a different arrangement of atoms in space. I.e. same number of atoms – but different structure.
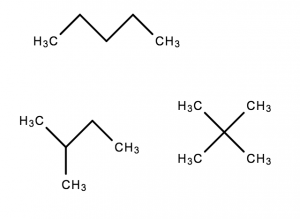
Example: C5H12
- These have the same molecular formula but have different structures
- Thus, they are different molecules, and have different properties
Note: the bonds without denoted C or H atoms are assumed to have Hydrogens attached in Chemistry
Geometric Isomers
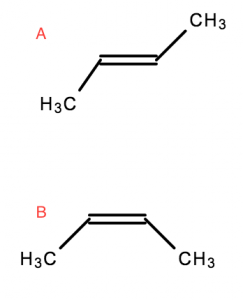
Geometric isomers are molecules that are locked into their spatial positions with respect to one another. This is due to a double bond or ring structure.
- Both of these molecules are But-2-ene
- However,
Ais Trans-But-2-ene, or (E)-But-2-ene Bis Cis-But-2-ene, or (Z)-But-2-ene
Trans (E) = Opposite
Cis (Z) = The same
Optical Isomers
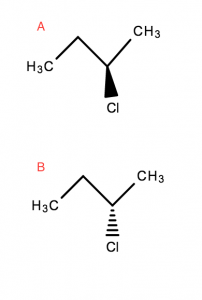
Optical isomers are molecules that differ three-dimensionally by the placement of substituents around one or more atoms in a molecule.
A‘s bond to Cl is coming towards the read in the third dimension- Whereas
B‘s bond to Cl is moving away, into the screen/page in the third dimension
Optical isomers are also enantiomers. These molecules differ in only one characteristic – i.e. how they interact with polarised light.
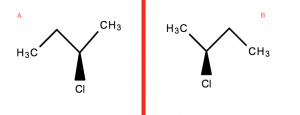
Optical isomers are mirror images that are non-superimposable on each other.
The red line representing a mirror
- From above, rotating B (A’s Mirror image) in any direction will not allow you to superimpose it directly on top
- The Cl atom in B will be facing the other way when rotated
This introduces the idea of Chirality. This is covered in the second part of this series on Isomers.





You must be logged in to post a comment.